A protein discovery could lead to healthier aging. Depositphotos –
A protein discovery could lead to healthier aging. Depositphotos –
Proteins are versatile macromolecules involved in pretty much every cellular process, including cellular repair and maintenance. Maintaining cellular fitness and function requires continuous protein balance or homeostasis. This involves ensuring proteins are in the correct concentration, shape, and location in the cell and removing those that are damaged or ‘incorrectly created.’
The problem is that, as we age, the body’s ability to clear away these dysfunctional proteins diminishes, which can lead to a harmful accumulation in the brain that has been linked to diseases like Alzheimer’s and Parkinson’s. A new study by researchers at McMaster University in Ontario, Canada, has identified a previously unknown cell-protecting function in a class of proteins that might be used to achieve healthy aging and treat neurodegenerative diseases.
“If the cells are experiencing stress because this protein aggregation has started, the endoplasmic reticulum, which is where proteins are made and then released, gets the signal to stop making these proteins,” said Bhagwati Gupta, a McMaster biology professor and the study’s corresponding author. “If it can’t correct the problem, the cell will die, which ultimately leads to degeneration of the neurons and then neurodegenerative diseases that we see.”
In previous studies, McMaster researchers had identified the protein mesencephalic astrocyte-derived neurotrophic factor (MANF) as protecting against cellular stress. In the present study, they were concerned with understanding how this happened. So, they turned to science’s favorite roundworm, C. elegans, and manipulated the amount of MANF the animal produced.
“We could literally see where MANF was expressed in the worms because they are translucent,” said the study’s lead author, Shane Taylor, who worked on the research as part of his PhD while at McMaster. “We could see it in all different tissues. Within these tissues, MANF was present in structures known as lysosomes, which are associated with lifespan and protein aggregation.”
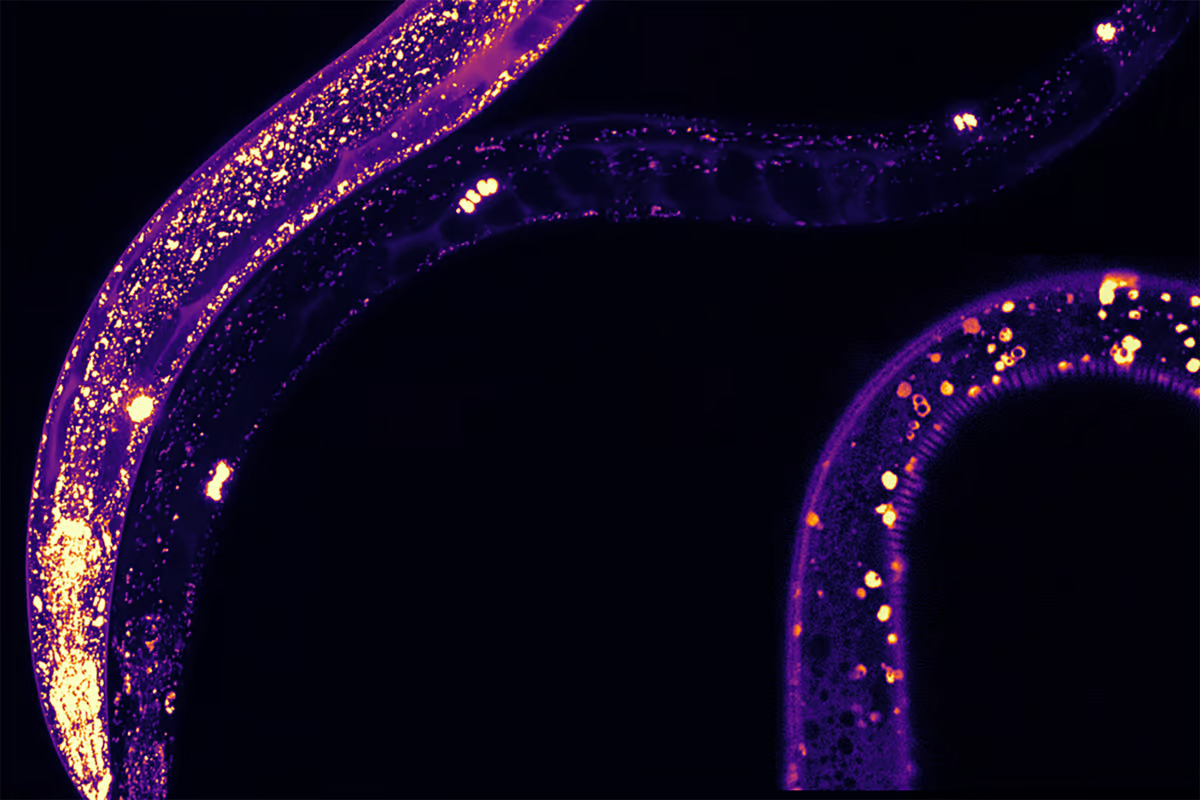
The researchers discovered that MANF plays a key role in a cell’s disposal process, helping to break down accumulated proteins and keeping cells debris-free and healthier. Increasing MANF levels in C. elegans activated a natural cellular clean-up system, helping them function better for longer. An overexpression of MANF in the worms extended their lifespan, reduced protein clumping, and protected neurons.
What implications do these findings have? That’s the question that New Atlas asked of the study’s senior researcher, Professor Bhagwati Gupta.
“[O]ur findings on the MANF protein have potential implications for neurodegenerative diseases such as Alzheimer’s and Parkinson’s,” Gupta said. “These diseases are often characterized by the accumulation of misfolded proteins and cellular stress, where MANF’s role as a protector against endoplasmic reticulum (ER) stress could indeed be relevant.
“Furthermore, while our research primarily focused on cellular mechanisms (i.e., processes happening inside cells), the broader implication of MANF’s activity could extend to aging,” continued Gupta. “Cellular homeostasis and the management of misfolded proteins and cellular debris are crucial aspects of aging, and MANF’s ability to enhance the cell’s natural protective mechanisms may contribute to healthier aging.”
The researchers are confident that MANF’s effects will translate to humans.
“Although our research focused on worms, the findings uncover universal processes,” Taylor said. “MANF is present in all animals, including humans. We are learning fundamental and mechanistic details that could then be tested in higher systems.”
More research is needed to develop MANF into a potential therapy, and the researchers particularly want to look at what other players the protein interacts with.
“The central idea of aging research is basically ‘can we make the processes better and more efficient’,” said Gupta. “By understanding how MANF works and targeting its function, we could develop new treatments for age-related diseases. We want to live longer and healthier. These kinds of players could help that.”
The study was published in the journal Proceedings of the National Academy of Sciences (PNAS).
Source: McMaster University
–