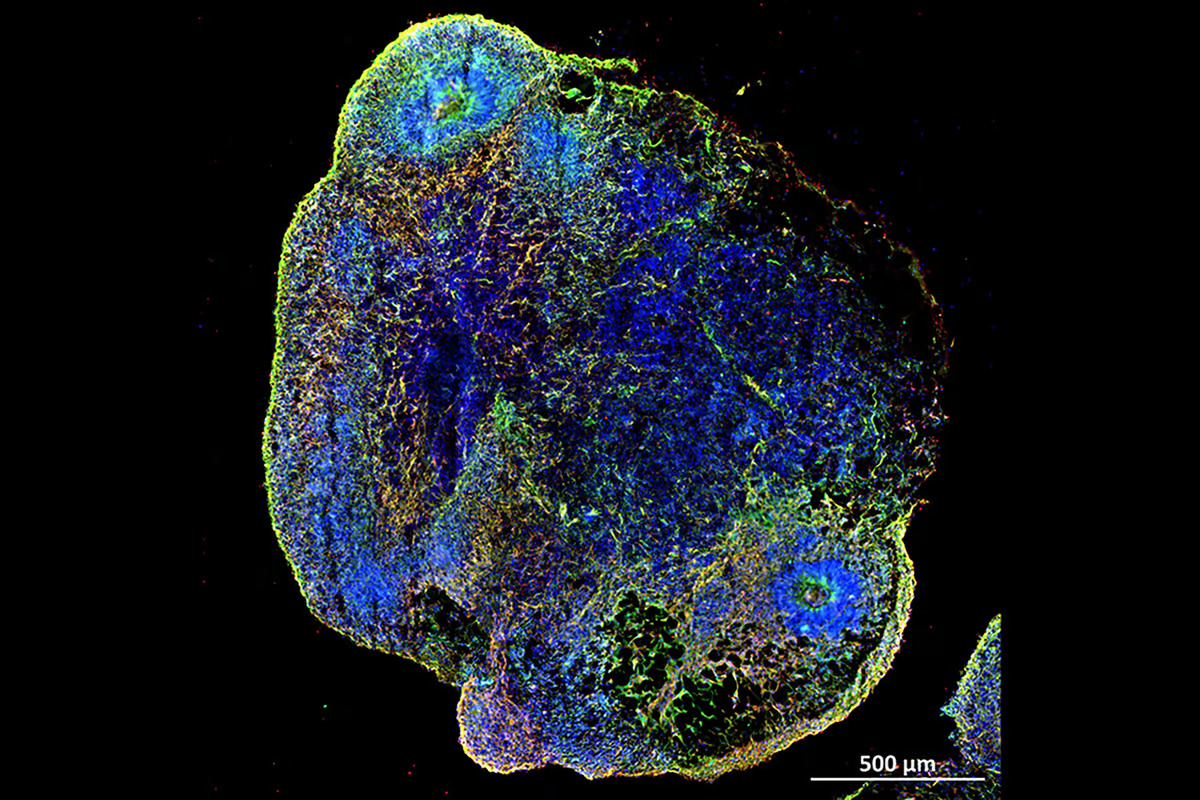
 Researchers have used lab-grown brain organoids, such as the one pictured here, to identify drugs to reverse premature brain aging caused by COVID-19 infection. AIBN/UQ
Researchers have used lab-grown brain organoids, such as the one pictured here, to identify drugs to reverse premature brain aging caused by COVID-19 infection. AIBN/UQ
–
Although it’s primarily a respiratory viral pathogen, in the post-acute phase SARS-CoV-2 has been shown to produce a range of neurological complications. Long COVID, often associated with cognitive impairment or brain fog, is one notable complication that is supported by ample evidence of significant structural changes in the brains of patients with COVID-19.
While the role of senescent or ‘zombie’ cells – cells that have stopped dividing – in driving neurodegenerative diseases and the cognitive decline seen in aging is supported by research, their contribution to COVID-related brain aging is unknown. This prompted researchers from the University of Queensland’s (UQ) Australian Institute for Bioengineering and Nanotechnology (AIBN) to study the effect of different SARS-CoV-2 variants on brain tissue and search for drugs that might reverse the process.
“We found COVID-19 accelerates the presence of ‘zombie’ or senescent cells, which accumulate naturally and gradually in the brain as we get older,” said Julio Aguado, lead and corresponding author of the study. “Senescent cells are known to drive tissue inflammation and degeneration, leaving patients exposed to cognitive impairments like brain fog and memory loss.”
The researchers hypothesized that SARS-CoV-2-induced senescence in the brain was associated with the neuroinflammatory effects of the virus during the acute phase. To test their hypothesis, they analyzed the brains of patients who’d died following severe COVID-19 or from non-infectious, non-neurological causes. They found an over sevenfold number of p16 protein-positive cells in the brains of COVID-19 patients compared to controls. Cellular senescence is often characterized by the expression of p16, and the findings suggested a potential role for SARS-CoV-2 in triggering cellular senescence, which could contribute to cognitive decline and accelerate the neurodegenerative processes associated with long COVID.
The researchers then generated brain organoids, lab-made mini-brain models, from embryonic stem cells and physiologically aged the organoids for eight months before testing the efficacy of senolytics or drugs that clear away senescent cells.
“We used the brain organoids to screen a range of therapeutics, looking for any capable of removing those senescent cells,” Aguado said.
They identified four drugs that selectively eliminated senescent cells: navitoclax, ABT-737, fisetin, and a combination of dasatinib and quercetin (D+Q). Navitoclax and ABT-737 inhibit the Bcl-2 protein, which induces apoptosis or programmed cell death in senescent cells. Fisetin and D+Q pass through the blood-brain barrier and clear senescent cells from the brain. The aged organoids were exposed to two doses (one every two weeks) of either navitoclax, ABT-737, or D+Q, after which they were subjected to bulk RNA sequencing analysis.

–
Compared to navitoclax and ABT-737, D+Q had a more broad-spectrum effect, mitigating multiple proinflammatory pathways characteristic of cellular senescence. In addition to acting as a senolytic, D+Q treatment on nine-month-old organoids reverted their gene expression age to levels comparable to eight-month-old counterparts. Gene expression changes caused by D+Q treatment were positively correlated with characteristics of lifespan-extending interventions such as caloric restriction, indicating the drug’s health-promoting role in targeting cellular senescence. In short, the treatment rejuvenated the organoids’ brain tissue.
Further to normal brain aging, the researchers infected the brain organoids with variants of SARS-CoV-2 and found that they led to a significant increase in cellular senescence, particularly the Delta variant. Treating the infected organoids with senolytics showed a marked loss of SARS-CoV-2 viral RNA expression.
The researchers then moved to experiments on mice infected with the SARS-CoV-2 Delta variant. Treatment with fisetin or D+Q significantly improved the survival of mice compared with controls, extending median lifespans by 60%. All senolytic interventions resulted in a profound reduction in COVID-related disease features, especially in the D+Q-treated group, including a reduction in p16 and proinflammatory cytokines. As with the organoid experiments, the researchers found that mice treated with senolytics had significantly lowered viral gene expression compared with untreated mice and that the senescence gene expression of infected mice was reduced to levels comparable to those of uninfected brains.
“More research is needed to fully understand the mechanisms at play, but this study marks a significant step forward in our knowledge of the intricate relationship between viral infections, aging and neurological well-being,” Aguado said. “Long term, we can expect widespread use of these drugs to treat persistent post-acute infection syndromes caused by viral infections like COVID-19.”
The researchers say using brain organoids enabled them to undertake ethical research that would be practically difficult in human subjects, and the same method could be used to study other senescence-related neurodegenerative diseases.
“Our study beautifully demonstrates how human brain models can accelerate the pre-clinical screening of therapeutics – while also moving towards animal-free testing – with potentially global impacts,” said Ernst Wolvetang, one of the study’s co-authors. “This same method of drug screening could also help Alzheimer’s research and a whole host of neurodegenerative diseases where senescence is a driver.”
The study was published in the journal Nature Aging.
Source: University of Queensland
–