Creating an organ as close as possible to the real thing is essential for research exploring disease pathology and testing new drugs. The brain presents particular challenges, including the fact that neurons grown in the lab have to form functional connections, and the brain tissue needs to support a complex but delicate architecture.
University of Wisconsin–Madison (UW-Madison) researchers have successfully 3D-printed brain tissue that grows and functions like a typical brain.
“This could be a hugely powerful model to help us understand how brain cells and parts of the brain communicate in humans,” said Su-Chun Zhang, the study’s corresponding author. “It could change the way we look at stem cell biology, neuroscience and the pathogenesis of many neurological and psychiatric disorders.”
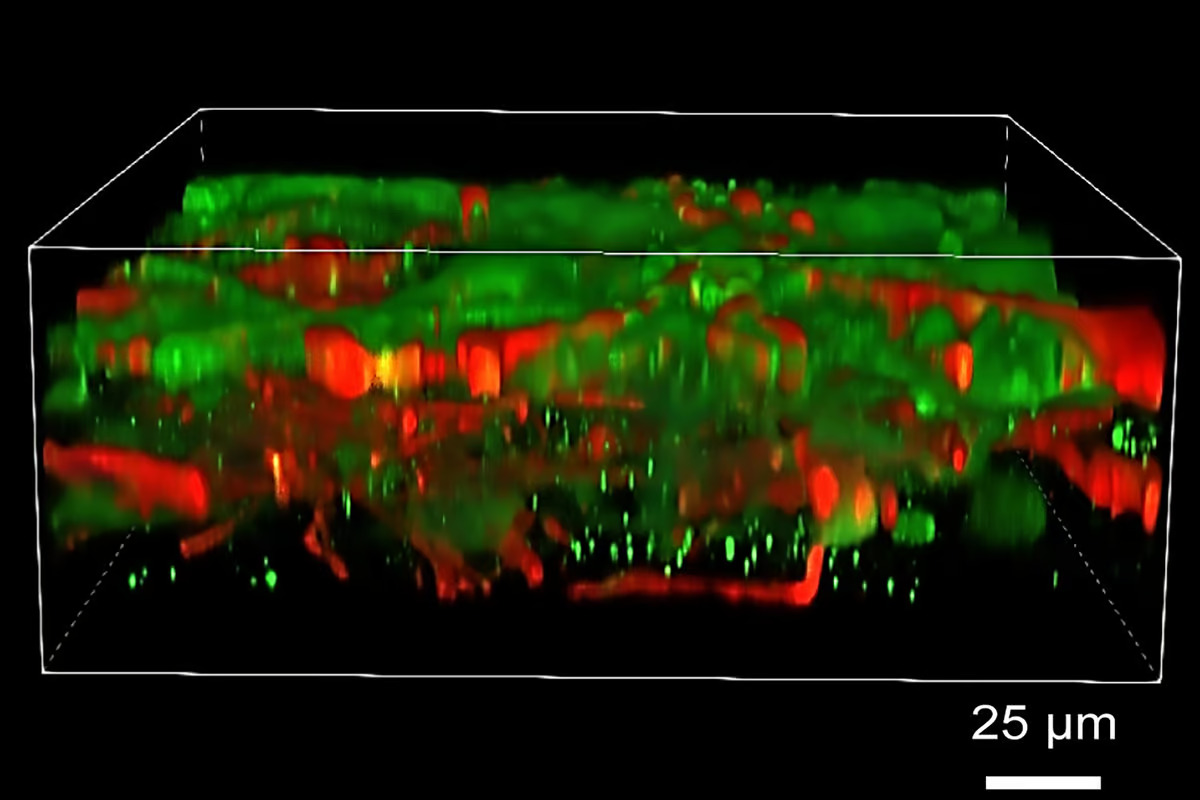
The researchers aimed to construct layered neural tissue in which neural progenitor cells (NPCs) mature and form connections (synapses) within and across layers while maintaining structure. They chose a fibrin hydrogel consisting primarily of fibrinogen and thrombin as the ‘bio-ink’, or biomaterial used for tissue printing, because it’s biocompatible with neural cells. Both fibrinogen and thrombin play a role in blood clotting.
The fibrin gel’s high viscosity made it difficult to print, so the researchers mixed it with a hyaluronic acid hydrogel, noting that a high number of NPCs placed into the mixture survived and matured. Adding another hydrogen made their bio-ink softer than those used previously.
Rather than using the traditional 3D printing approach of vertically stacked layers, which requires a stiff bio-ink printed in thick layers, the researchers created patterned tissue by printing one thin layer or band of cell-infused gel next to another horizontally. To prevent the mixing of printed bands, thrombin was used as a crosslinking agent immediately after the mixture was deposited.
Although the printed cells stayed within their designated layers, the neurons formed functional synaptic connections in and between layers within two to five weeks after printing.
“The tissue still has enough structure to hold together, but it is soft enough to allow the neurons to grow into each other and start talking to each other,” Zhang said. “Our tissue stays relatively thin, and this makes it easy for the neurons to get enough oxygen and enough nutrients from the growth media.”
The researchers tried printing brain tissue using different combinations of cells in the bio-ink.
“We printed the cerebral cortex and the striatum, and what we found was quite striking,” said Zhang. “Even when we printed different cells belonging to different parts of the brain, they were still able to talk to each other in a very special and specific way.”

The researchers say their approach offers precision over the types and arrangements of cells that organoids and other printing methods don’t. And the printing technique doesn’t require special equipment or culturing methods to keep the tissue healthy, meaning that it should be accessible to many labs.
“Our lab is very special in that we are able to produce pretty much any type of neurons at any time,” said Zhang. “Then we can piece them together at almost any time and in whatever way we like. Because we can print the tissue by design, we can have a defined system to look at how our human brain network operates. We can look very specifically at how the nerve cells talk to each other under certain conditions because we can print exactly what we want.”
There are plans to refine the bio-ink and equipment to allow for specific cell orientations within their printed tissue.
“Right now, our printer is a bench-top commercialized one,” said the study’s lead author, Yuanwei Yan. “We can make some specialized improvements to help us print specific types of brain tissue on demand.”
The researchers say their printed brain tissue could be used to study cell-cell signaling in Down syndrome, interactions between healthy and Alzheimer’s-disease-affected tissue, test new drug candidates, or simply watch how the brain develops.
“In the past, we have often looked at one thing at a time, which means we often miss some critical components,” Zhang said. “Our brain operates in networks. We want to print brain tissue this way because cells do not operate by themselves. They talk to each other. This is how our brain works, and it has to be studied all together like this to truly understand it.”
The study was published in the journal Cell Stem Cell.
Source: UW-Madison
–